T b the amount the boiling point increases. Either vapor pressure boiling point elevation freezing point depression or most commonly osmotic pressure is measured and the appropriate equation is used to calculate the concentration molarity from.

Freezing Point Depression Boiling
Boiling-Point Elevation and Freezing-Point Depression.

Boiling point elevation freezing point depression worksheet answers. CHEMISTRY COLLIGATIVE PROPERTIES WORKSHEET m 152g 142 g mole0875 kg of solvent 122 m m. The amount of the elevation of the boiling point of a solution is given by Tb ikbm where kb is the molal boiling point elevation constant of the solvent and m is the molal concentration of the solute. Like the boiling point elevation eect the freezing point depression eect is too small.
Discover learning games guided lessons and other interactive activities for children. A solution is prepared by dissolving 270 g of urea NH 2 2 CO in 1500 g of water. 351 T b k b m i.
The technique is insensitive and only useful for low molecular weight polymer eg M N less than 20000 gmol. Boiling Point ElevationFreezing Point Depression Worksheet Name. Freezing Point Calculation Boiling Point Calculation.
Freezing Point Depression T i k f m solute. 35B 36 Assume you have a 570m solution that raised the boiling point of the solution by 1620C. We cannot express the concentration of the solution in molarity because it changes with temperature whereas molality is temperature independent.
The amount of the depression of freezing point is given by Tf -ikfm where kf is the molal freezing point. What is the molal boiling point elevation constant KJ of the solution. Molal freezing point depression constant Kf of the solution.
Change in the freezing point of the solution is T f 29103 C. Discover learning games guided lessons and other interactive activities for children. Because the addition of a nonvolatile solute to a volatile solvent lowers the vapor pressure of the solution relative to the pure solvent the vapor pressure of the solution will be lower than the pure solvent at any temperature.
The molal elevation of freezing point constant is defined as the depression of freezing point produced when one mole of solute is dissolved in 1 kg of solvent. Ad Download over 20000 K-8 worksheets covering math reading social studies and more. _____ Student ID_____ Work in groups on these problems.
The equation used to calculate the increase in the boiling point is. Boiling Point Elevation T i k b m solute. In boiling point elevation and freezing point depression we deal with the systems whose temperature is not constant.
Looking at the formula for the boiling point elevation and freezing point depression we can see similarities between the two. Calculate the freezing point of the solution. T f -K f m K f 1855 from table above m 0262 m T f -1855 0262 -0486 o C.
Assume the K f of water is -186Cm. Freezing point depression is a colligative property observed in solutions that results from the introduction of solute molecules to a solvent. FREEZING POINT DEPRESSION BOILING POINT PROPERTY ELEVATION m 92g 180 g mole0255 kg of solvent 20 m.
The freezing-point depression Delta T_f can be calculated in a similar manner Delta T_t K_f middot m in which m is the molality of the solution and K_f is the molal freezing-point-depression constant for the solvent. Osmotic Pressure i MRT. M molality moles solute kg solvent K b boiling point elevation constant water K b 052 o Cm D T f change in freezing point.
Calculate the boiling point of the solution. If you use 368mol of sucrose C 12 H 22 O 12 and dissolve this into 250kg of water what will be the change in the freezing point of your solution. We found some Images about Freezing Point Depression And Boiling Point Elevation Worksheet Answer Key.
The freezing points of solutions are all lower than that of the pure solvent and is directly proportional to the molality of the solute. Raoult discovered that the addition of. Boiling-Point Elevation and Freezing-Point Depression.
Colligative properties boiling point elevation worksheet Name. Freezing point depression and boiling point elevation are examples of colligative properties. I vant Hoff factor.
Normal Freezing Point of Water 000 o C. Calculate the freezing point depression. Camphor CloH160 has a molal freezing point depression.
K f freezing point depression constant water K f -186 o Cm Normal Boiling Point of Water 100. Cyclohexane has a freezing point of 660 degree C and a K_f of 200 degree Cm. Now The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy.
54 Osmotic Pressure Another colligative property is osmotic pressure. Ad Download over 20000 K-8 worksheets covering math reading social studies and more.
Colligative Colloids Solution Osmosis

Colligative Properties Worksheet Colligative Properties Worksheet 1 If I Add 45 Grams Of Sodium Chloride To 500 Grams Of Water What Will The Melting Course Hero

Freezing Point Depression And Boiling Point Elevation Wksht Quia

Boiling Point Elevation Freezing Point Depression Worksheet Pdf Course Hero
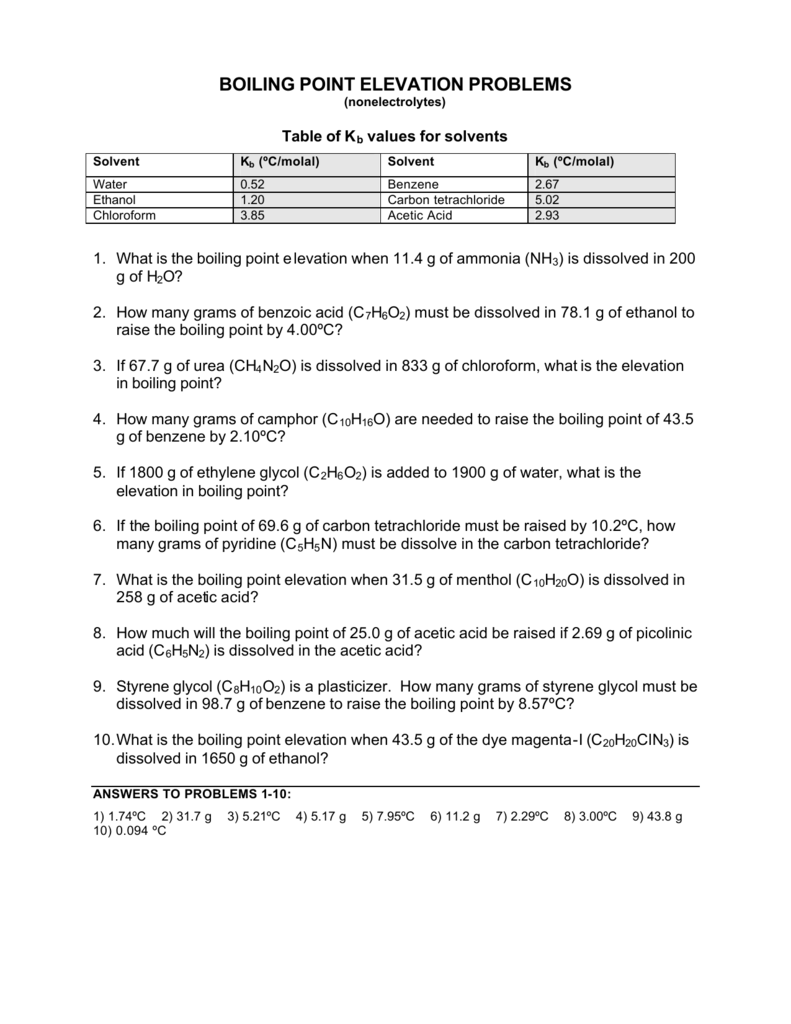
Boiling Point Elevation Problems
Colligative Properties Freezing Point Depression Worksheet For 10th Higher Ed Lesson Planet

Colligative Properties Of Water Worksheet Boiling Point Elevation Freezing Point Depression Worksheet Name P 1 If You Use 3 68mol Of Sucrose C12h22o12 Course Hero
Http Chan Chemistry Bps Weebly Com Uploads 3 7 9 2 37922623 Colligative Properties Worksheet Key Pdf
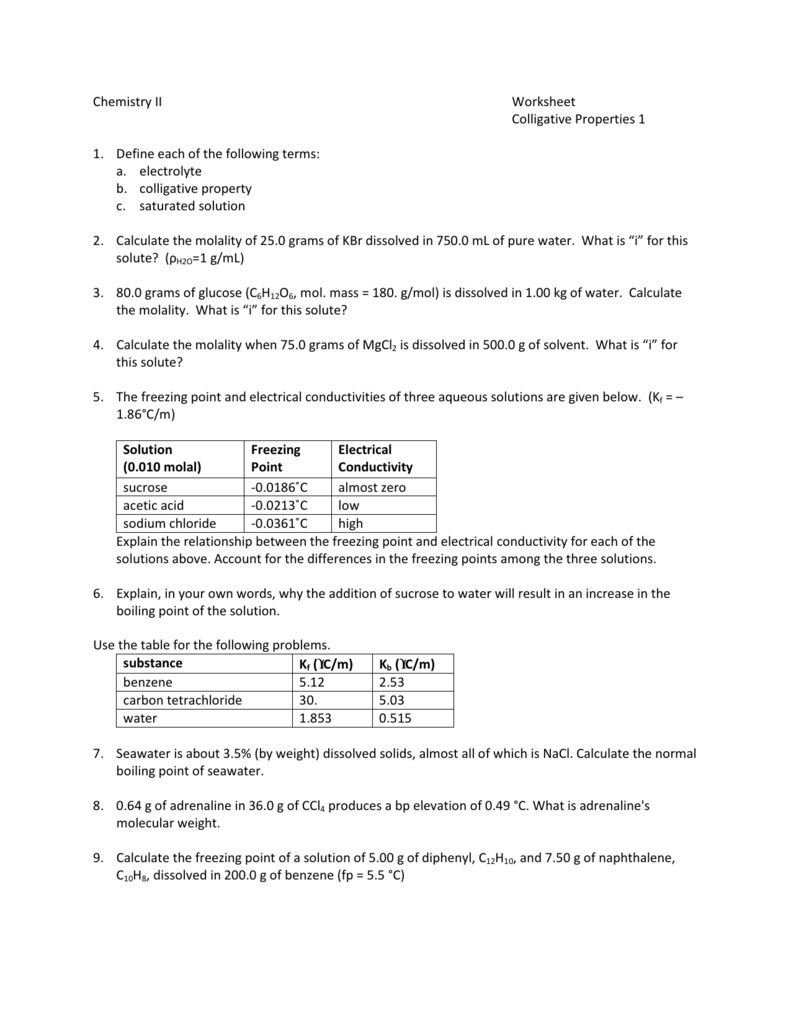
Chemistry Ii Worksheet Colligative Properties 1 1 Define Each Of The
Freezing Point Depression Pre Lab Answers
Http Garzscience Weebly Com Uploads 2 5 4 7 25474030 Colligative Properties Key Pdf

Freezing Point Depression Boiling Point Elevation

Colligative Properties Worksheet Ii Answer Key 11 12

Chemistry Colligative Properties Worksheet

Boiling Point Elevation Freezing Point Depression Worksheet Pdf Course Hero

Colligative Properties Worksheet Key Name Ki Colligative Properties Worksheet Date 2 What Is The Normal Boiling Point Of A 0 122 M Aqueous Solution Course Hero

F P Depression And B P Elevation Calculations Worksheet By John Mcintosh

Freezing Point Depression And Boiling Point Elevation Freezing Point Depression And Boiling Point Elevation Problems Name Block 1 Determine The Course Hero

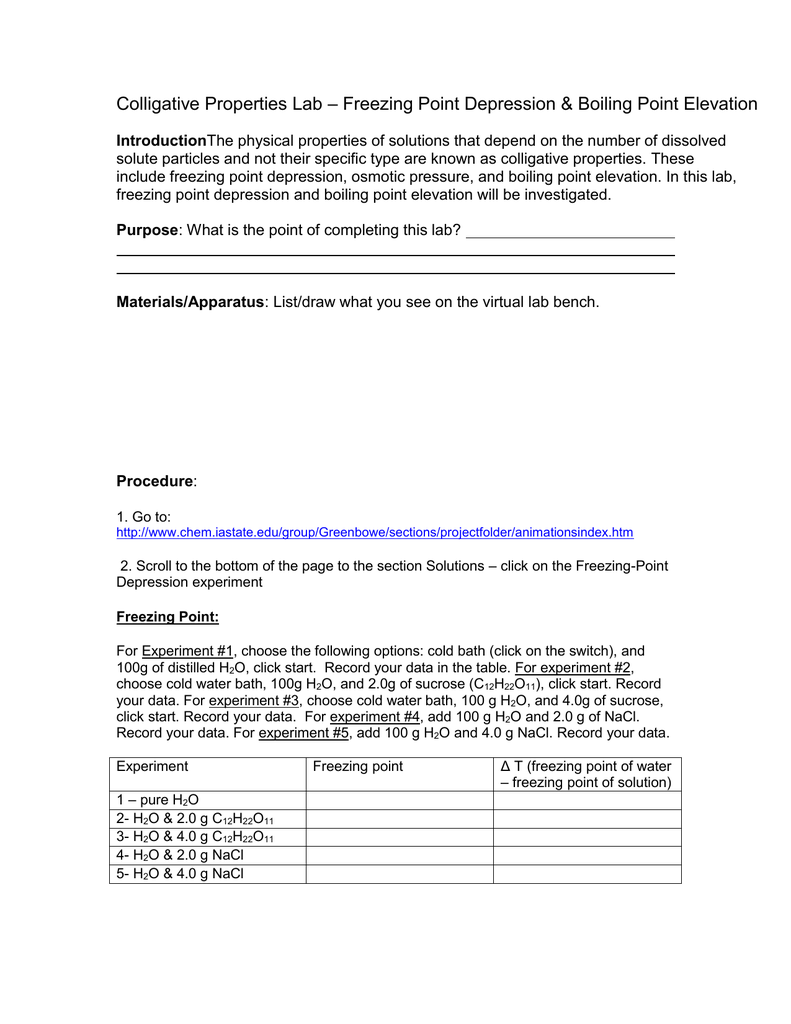

0 comments:
Post a Comment